9.5 Carnot's Theorem
All reversible engines working between the same limits of temperature must have the same efficiency.
Proof (A): If not, let an engine S be more efficient than an engine R (Fig. 9.3). This means that by drawing the same quantity of heat from the source, the work WS produced by S is greater than the work WR produced by R. Accordingly, S can be made to drive R backwards.
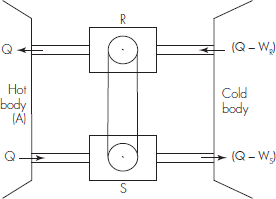
Fig. 9.3 Scheme representing Carnot's theorem
Let the quantity of heat drawn by S from the source be Q. Then, the quantity of heat given by S to the refrigerator is (Q − Ws). Let the quantity of working substance in R be so adjusted that in each reverse ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

