28.2. High Pressure for Freezing: Principles and Equipment
Figure 28.1 shows the phase diagram of water. Ice I, the common ice at atmospheric pressure, is stable up to 210 MPa. Above this pressure, different ice polymorphs, from ice II to ice XV, are thermodynamically stable depending on the pressure/temperature coordinates (Bridgman, 1912; Salzmann et al., 2006, 2009).
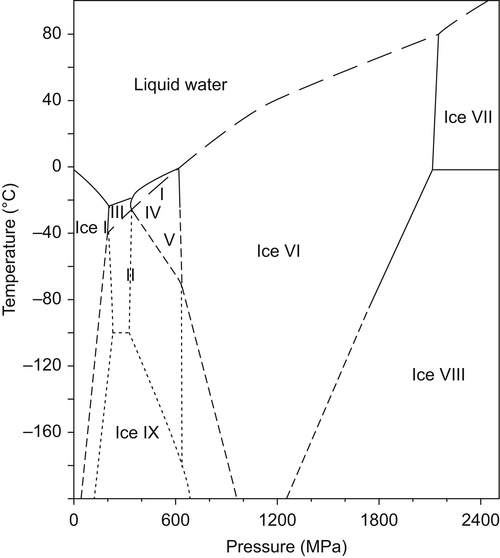
The freezing point of water decreases with pressure up to 210 MPa. The opposite was observed above this level for ice types other than ice I (see Figure 28.1). According to the Clausius–Clapeyron equation,
(28.1)
where T is temperature ...
Get Emerging Technologies for Food Processing, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

