 Appendix K
Appendix K
Basic Kinetic Theory of Gases
The ideal gas law states that
![]()
where P is the pressure, V is the volume of one mole of gas, R is the gas constant (1.98 cal/mol-K, or 82 atm-cm3/mol-K), T is the absolute temperature in K, Nau is Avogadro's number (6.02 × 1023 molecules/mole), and k is Boltzmann's constant (1.38 × 10−23 J/K, or 1.37 × 10−22 atm-cm2/K). Since real gases behave more and more like the ideal gas as the pressure is lowered, Eq. l is valid for most vacuum processes. We can use Eq. l to calculate the molecular concentration n (the number of molecules per unit volume):
![]()
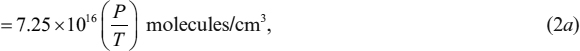
where P is in Pa. The density ρd of a gas is given by the product of its molecular weight and its concentration:
![]()
The gas molecules are in constant motion and their velocities are temperature dependent. The distribution of velocities is described by the Maxwell-Boltzmann distribution law, which states that for a given speed υ,
where m is the mass of a molecule. This equation states that if there are n
Get Semiconductor Devices: Physics and Technology, 3rd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

