Chapter 4
Surface Tension Determination
INTRODUCTION
Surface tension is a phenomenon in which the surface of the liquid is in contact with the gas. Surface tension is defined as the force acting at right angles to a line of unit length present in the surface. This is denoted by ‘γ’.
where W = work; F = force; d = distance.
Therefore,
The SI units for the surface tension are newton/meter or dyne/cm.
At a liquid surface, the molecule is not completely surrounded by other molecules.
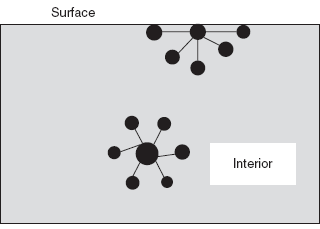
Surface tension phenomenon
The surface tension of a liquid is the energy required to increase the surface area which increases ...
Get Pharmaceutical Analysis now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

