9.6 Efficiency of A Carnot's Engine Is Independent of Nature of the Working Substance
Let us have two Carnot’s engines X and Y (Fig. 9.5) working with two substance x and y between two limits of temperature θ1 and θ2 (θ1 > θ2) independently.
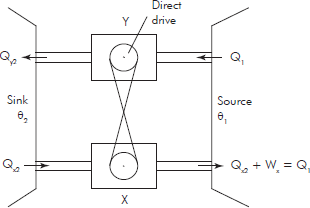
Fig. 9.5 Carnot's engine
Let the engine X draw Q1 quantity of heat at θ1 and reject Qx2 quantity of heat at θ2 after doing Wx amount of work when running independently. Let the engine Y draw Q1 quantity of heat at θ1 and reject Qy2 quantity of heat at θ2 after doing Wy amount of work when running independently
Then by the first law,
and
so that
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

