7.1. Introduction
In Joule's experiment, a quantity of compressed gas was allowed to expand into a vacuous space without doing external work. No change of temperature of the gas was detected after expansion. The consequence of this was
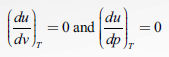
Thus at constant temperature, u is not a function of p and v. It must, therefore, be a function of T only.
The thermal capacity of the apparatus employed was very large in comparison with that of the gas experimented upon. To detect any thermal effect that might reasonably have been expected, a more delicate arrangement was necessary. Joule in collaboration with Thomson carried out extensive series of researches ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

