Solved Problems
Q1. Calculate the root mean square (RMS) velocity of an oxygen molecule at 0°C given the gram molecular mass of the gas is 32 gm and the molar gas constant R is 8.32 × 107 ergs per mole per degree. At what temperature will the RMS velocity of the gas have the same magnitude as the velocity of sound in air, that is, 332 metres per sec?
Ans. The RMS velocity
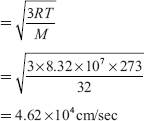
Let the required temperature be T K, then
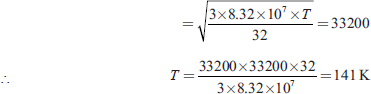
Q2. At what temperature will the average speed of hydrogen molecules be the same as that of nitrogen molecules kept at 35°C? ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

