Solved Problems
Q 1. The melting point of pure acetic acid is 16.6°C and this is raised by 0.0244°C per one atmosphere increase of pressure. If 1 gm of acetic acid occupies 0.00079 litre in the solid state and 0.00095 in the liquid state at its melting point, calculate the latent heat of fusion in work units (litre-atmosphere) per gram.
Ans.
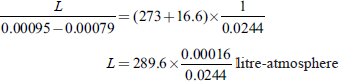
Q 2. Calculate the change in Cp of water at 22°C.
Ans. We know
Here,
Mean value of
Hence,
Q 3. Calculate the specific heat of copper at constant volume ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

