Solved Problems
1. Chromium has BCC structure. Its atomic radius is 0.1249 nm. Calculate the free volume/unit cell.
(Set-4–May 2007), (Set-4–Sept. 2006)
Sol: Given data are
Atomic radius of chromium, r = 0.1249 nm.
Free volume/unit cell = ?
If ‘a’ is the BCC unit cell edge length, then the relation between ‘a’ and ‘r’ is
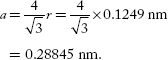
Volume of unit cell, V = a3 = (0.28845)3 nm3
= 0.024 nm3
Number of atoms in BCC unit cell = 2
Hence volume of atoms in unit cell, ![]()
Free volume/unit cell = V – v = 0.00767 nm3
2. Lithium crystallizes ...
Get Engineering Physics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

