Chapter 11
Chemical Equilibrium
11.a. The Law of Mass Action
Chemical reactions are reversible in nature i.e. the reaction can proceed simultaneously in the forward and reverse directions. In such case, the sign ![]() is used to indicate that the reaction is reversible e.g.,
is used to indicate that the reaction is reversible e.g.,
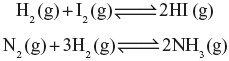
Chemical reactions are affected by changes in temperature, pressure and concentration. By investigating the effect of mass (concentration) on the course of chemical reactions, Guldberg and Waage, in 1867, proposed the law of mass action as ‘at constant temperature the rate or velocity ...
Get Chemical Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

